What Does an Atom Look Like?
Most recent answer: 10/22/2007
- Anonymous
Dear Winnie:
We have a very long related thread here:
http://van.physics.illinois.edu/qa/listing.php?id=1295.
A link to a picture is included in http://van.physics.illinois.edu/qa/listing.php?id=1280.
Also see http://van.physics.illinois.edu/qa/listing.php?id=1195.
The big problem with answering "what does an atom look like" is that in order to look at something, we normally shine light on it and observe the light that bounces off. Visible light has a wavelength that is much bigger than atoms. It is impossible to make out small details of objects by probing them with waves of large size -- the waves simply flow around the small object. Shorter-wavelength light may be used, but the energy per photon increases as the wavelength shortens. As it turns out, when the light has a wavelength comparable to the size of the atom or smaller, it has enough energy to change the atom by moving the electrons around. This is one consequence of Heisenberg's Uncertainty Principle. We cannot say where the electrons are and how fast they are moving at the same time, and hence we cannot draw a picture with a little electron on one place sitting on a circular orbit. The fuzzy picture at the bottom of the page linked to is closer to the truth, describing the probabilities of finding electrons, depending on where you look for them.
The nucleus, containing protons and neutrons, has a similar problem, but it is much much smaller than the electron cloud, and hence the energy of a photon needed to resolve structure is much higher. Heisenberg's Uncertainty Principle applies to neutrons and protons as well, and so a fuzzy nucleus isn't such a bad representation either. But for show in a classroom, the clear pictures showing the details get across the important points that atoms consist of protons, neutrons, and electrons, that the protons and neutrons are in the nucleus and the electrons are found at larger radii.
Tom
(published on 10/22/2007)
Follow-Up #1: What Atoms look like
- Ellen Wetmore (age 40)
Groton MA
It's great to hear you have such a curious 6 year old! When he asks about what they look like, there are at least two sides to the question. One is what shape they are and the other is their color.
It turns out that visible light is no good for seeing the shape of atoms, because its wavelength is way bigger than the atom. In order for the shape to be clearly seen, an object has to be larger than the wavelength of light. The wavelength of visible light is about 400 nanometers to 700 nanometers (a nanometer is one billionth of a meter or about 1/15,000 to 1/200,000 of a human hair) but the largest atom is around 270 picometers in diameter or more than 1000 times smaller than visible light. So, while individual atoms can reflect visible light, because atoms are so much smaller than the light itself, you will never be able to see any more than a fuzzy dot.
In order to clearly visualize atoms, a scanning tunneling microscope is used which is a microscope that uses electrons instead of light to see very small objects. The resolution of these microscopes is good enough so that individual atoms can be seen as bumps. Some of the most famous pictures of individual atoms is the letters "IBM". Beyond actual images, you could take a look at some earlier responses to what atoms look like and some of the links provided . One picture of the letters "IBM" spelled from atoms is below.
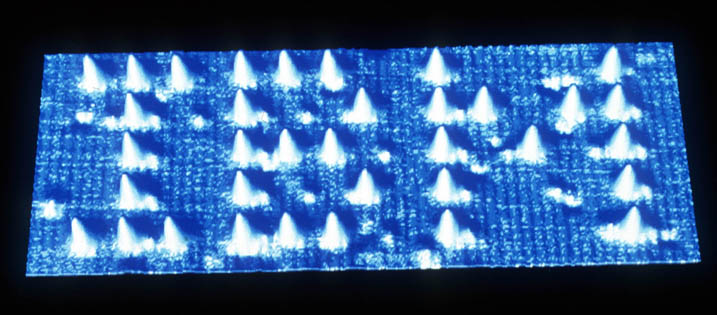
As for the color, atoms can have visible colors, altough that's hard to see unless you have a lot of them. By an object's color, people sometimes mean the color of the light that bounces of it, and sometimes they mean the color of the light that it emits.
Some atoms have very clear visble colos of emission, such as the sodium atoms that emit yellow light in some street lights. So when you see the yellow glow from a streetlamp, that could be the color of sodium atoms! Some others don't usually emit visible light.
All atoms will scatter some of the light that hits them. Usually they scatter more blue light than red. The molecules (pairs of atoms) in the air do that, and that's why the sky is blue. Any one atom or molecule doesn't scatter much, though.
Just as atoms can emit light, they can also absorb light. The colors you see when light goes through a collection of atoms that can absorb are just the colors that that particular type of atom doesn't absorb.
Erik +Mike W.
(published on 03/12/2013)